Daily vaccination data is not being updated after June 30, 2021. To see current weekly vaccination rates and COVID-19 data in Minnesota, go here. To read frequently asked questions about COVID-19 vaccines in Minnesota, go here.
After months of trials, three coronavirus vaccines have been approved by federal health officials and Minnesota's vaccination program is underway. Here is information about the progress of vaccinations in Minnesota and answers to questions you may have about vaccines and the state's strategy to vaccinate millions of Minnesotans. Jump to the FAQ.
Frequently Asked Questions
Which vaccines have been approved?
While more than 50 vaccine candidates have progressed to clinical trials with humans, two have been approved for emergency use by the U.S Food and Drug Administration. A vaccine developed by Pfizer and BioNTech was approved for those age 16 and older by the FDA on Dec. 11, and a second vaccine produced by Moderna was authorized for adults over the age of 18 Dec. 18. A single-dose vaccine produced by Johnson & Johnson was approved for those age 18 and older Feb. 27. A fourth vaccine developed by the University of Oxford and AstraZeneca could be ready for review in coming months.
The FDA on May 10 authorized use of the Pfizer-BioNTech vaccine for children age 12 and older. Centers for Disease Control and Prevention (CDC) advisers endorsed the use of the Pfizer vaccine in kids as young as 12 on May 12 and the CDC quickly accepted their recommendations, paving the way for shots to begin.
How do the vaccines work?
The Pfizer/BioNTech and Moderna vaccines use messenger RNA to instruct cells to create a harmless piece of the virus that causes COVID-19. This piece of "spike protein" is a key feature of the virus (you've likely seen COVID-19 depicted as a spiky ball). The body recognizes this spike protein as foreign and triggers an immune response to fend off future infection.
The Johnson & Johnson and AstraZeneca vaccines are called viral vector vaccines, using the adenovirus — a strain of the common cold found in chimpanzees — to deliver a piece of that spike protein into cells. This prompts an immune response to inoculate the recipient against infection.
How are vaccines being distributed in Minnesota?
The FDA approved the Pfizer vaccine Dec. 11 and the Moderna vaccine on Dec. 18. The Johnson & Johnson vaccine, which was approved Feb. 27, began shipping to Minnesota in early March, state health officials said. By the end of March, Johnson & Johnson has said it expects to deliver 20 million doses to the U.S., and 100 million by summer. However, that timeline will likely change pending a "pause" in using the single-dose Johnson & Johnson COVID-19 vaccine.
The first doses of the Pfizer vaccine arrived in Minnesota at the Minneapolis VA Medical Center on Dec. 14. Vaccines are being shipped to "hub" medical facilities around the state. From there, they are being distributed to smaller clinics, or "spokes," that make doses available to providers. The federal government decides how many doses each state will receive weekly.
Who is able to get vaccinated?
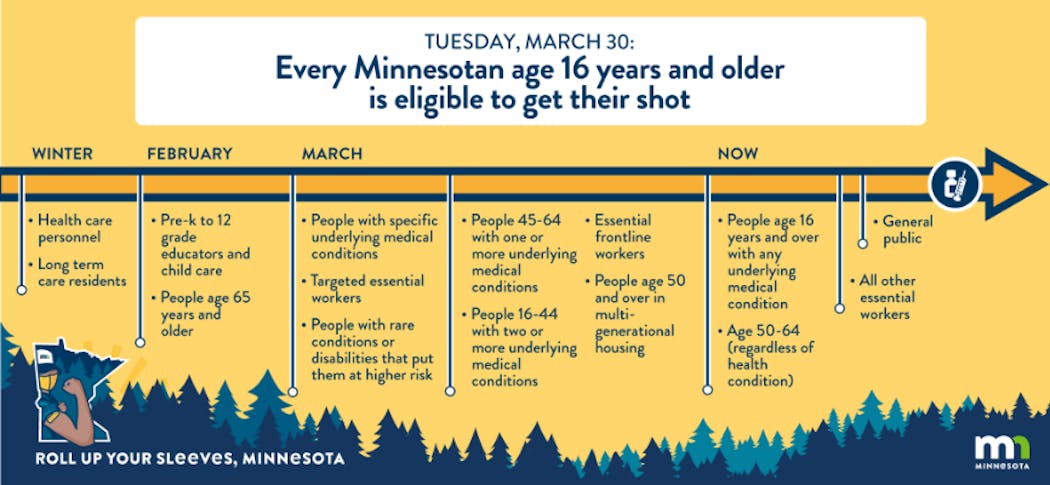
In Minnesota, first priority was given to front-line health care workers in COVID-19 hospital units, emergency departments and nursing homes along with paramedics, COVID-19 testing personnel and some public health workers. Residents in nursing homes were also in the highest priority group. President Joe Biden pledged in a March 12 prime-time address that all Americans would be eligible to receive a vaccine by May 1 and raised the possibility of beginning to "mark our independence from this virus" by the Fourth of July. The plan to administer vaccines in Minnesota has been divided into phases. A detailed breakdown can be found here.
- Phase 1a: Health care workers, residents and staff of long-term care facilities and those 65 years of age and older. Primary caregivers for people with complex medical needs or disabilities. Some vaccine doses were also made available to school teachers and child care workers.
- Phase 1b: All people with specific conditions, including: Sickle-cell disease, Down syndrome, those in active cancer treatment or immunocompromised from organ transplant, oxygen-dependent chronic lung and heart conditions, as well as people with rare conditions or disabilities that put them at higher risk. People ages 45 to 64 with one or more of the following high-risk medical conditions, or people ages 16 to 44 with two or more of these conditions, including: Active cancer, chronic kidney disease; chronic obstructive pulmonary disease (COPD); heart conditions, such as heart failure coronary artery disease, or cardiomyopathies; weakened immune system due to organ transplants, HIV, bone-marrow disease, chronic steroids for more than 30 days, immunodeficiency disease, or immunosuppressive medications; obesity (body mass index greater than 30); Pregnancy; Type 1 or 2 diabetes. Essential frontline workers, including: agricultural, Pre-K through Adult Basic and Community Education school staff or contracted school staff, child care staff at licensed and certified child care centers or programs, first responders, those who work in correctional settings, food-processing plant workers, food production, food retail, food service, judicial system, manufacturing, public transit and postal workers. People age 50 or older who live in multi-generational housing. Any remaining individuals covered in previous phases are also eligible to be vaccinated.
- Current phase: COVID-19 vaccines became available to all Minnesotans 16 and older beginning Tuesday, March 30, Gov. Tim Walz announced March 26. More information about the latest phase of the vaccination program can be found here. Some vaccine providers began offering appointments to children age 12 and older on May 13.
The state on Feb. 18 debuted a COVID-19 vaccine registration tool that is available to all Minnesota residents who have not yet been vaccinated. Enter your personal and contact information and you will be notified when they are eligible to receive the vaccine. It will also give senior citizens immediate access to a lottery for appointments at state-operated sites. More information about making an appointment can be found here.
How do I make an appointment to be vaccinated?
All Minnesotans who have not yet been vaccinated can register on the state's vaccine connector website to be notified when they become eligible for the vaccine. If you are eligible to be vaccinated, you can use this online locator map to find local providers administering the vaccine and register for the lottery for appointments on the vaccine connector site. More information about making an appointment can be found here.
How are the vaccines administered?
The Pfizer and Moderna vaccines require two doses, delivered via injection. The Pfizer vaccine requires a booster shot three weeks after the first dose, while the Moderna vaccine's booster shot is administered four weeks later. Johnson & Johnson's vaccine is a single shot. It's unclear what dosage schedule AstraZeneca will submit to regulators.
What are some of the differences between the vaccines?
Both the Pfizer and Moderna vaccines must be frozen for transportation and storage. Pfizer's vaccine is the most demanding, needing a storage temperature of -70 degrees Fahrenheit, which requires specialized freezers. Moderna has said its vaccine can be kept stable at around -20 degrees Fahrenheit for up to six months, closer to the temperature of a standard freezer.
Both the AstraZeneca and Johnson & Johnson vaccines can be stored in a refrigerator, making them easier to ship and to use in rural areas and developing countries than the frozen kind made by Pfizer and Moderna. AstraZeneca's vaccine is also significantly cheaper to manufacture, at roughly $3-$4 per dose.
How safe are the vaccines?
The first phase in any clinical trial is dedicated to making sure a drug is safe. Vaccines cannot be approved without passing this crucial step. According to the CDC, a clinical trial is paused whenever an "unexpected health event" is detected so that researchers can investigate any potential safety concerns. Minnesota health officials have said that they have confidence in the clinical trials conducted and the regulatory approval process so far.
The experimental vaccines have been tested in tens of thousands of volunteers so far, and serious side effects have not been reported. Health officials will be monitoring for side effects as more people get vaccinated, as well as for any potential longer-term issues.
On April 13, U.S. officials recommended a "pause" in using the single-dose Johnson & Johnson COVID-19 vaccine to investigate reports of rare but potentially dangerous blood clots. Health officials learned of 15 cases of a rare blood clot in conjunction with a platelet condition out of more than 8 million doses administered. All of the cases were found in women, three of whom died. Data presented to the Advisory Committee on Immunization Practices (ACIP) reinforced initial concerns that the risk seems to be greatest among women under 50. Federal health agencies lifted the pause on using the Johnson & Johnson vaccine April 22, after an advisory committee said the benefits more than offset the risks of the rare but serious blood clot problem.
Dr. Anthony Fauci, the nation's top infectious disease expert, has noted that people might feel achy or feverish right after the shot, or some soreness in the arm. Other temporary side effects reported by study participants included fatigue, headache and chills.
How effective are the vaccines?
Pfizer and Moderna have both reported that their vaccines reached 95% efficacy in clinical trials, far exceeding scientists' expectations. According to Fauci, the goal was to reach 75% efficacy with any COVID-19 vaccine, and the FDA said it would approve a vaccine with just 50% efficacy.
The FDA said Johnson & Johnson's vaccine offers strong protection against what matters most: serious illness, hospitalizations and death. One dose was 85% protective against the most severe COVID-19 illness, in a massive study that spanned three continents — protection that remained strong even in countries such as South Africa, where the variants of most concern are spreading. Researchers found no one who got the vaccine needed hospitalization or died.
AstraZeneca has reported 62% efficacy with its vaccine, though that rate appears to jump to 90% when administered with a stair-step dosage. Participants were given a half-dose for the first round of immunization followed by a full dose, and researchers noted the vaccine was more effective.
Are the vaccines effective immediately?
The Pfizer vaccine provides strong protection against COVID-19 within 10 days after the first dose, according to documents published by the FDA. Minnesota state epidemiologist Kris Ehresmann cautioned that it could take up to six weeks after the first dose for vaccines to provide full protection against COVID-19.
How long will the vaccines protect against infection?
We don't know yet. It's possible that these vaccines could provide long-lasting protection against the virus, or the protection could fade over time and require additional booster shots. The FDA said the Pfizer vaccine seems to provide protection for at least two months after the second and final dose.
Will I still need to wear a mask after vaccination?
The CDC eased indoor mask-wearing guidance for fully vaccinated people May 13, allowing them to safely stop wearing masks inside in most places. The CDC will also no longer recommend that fully vaccinated people wear masks outdoors in crowds. The new guidance still calls for wearing masks in crowded indoor settings like buses, planes, hospitals, prisons and homeless shelters. Gov. Tim Walz signed an executive order Friday that will lift Minnesota's statewide mask mandate, but businesses and municipalities may continue to require masks. MDH recommends that those who are not vaccinated continue to wear masks. Face covering requirements will remain in place at schools and child-care settings, as well as at medical facilities and on public transportation.
Can children be vaccinated?
The FDA on May 10 authorized the use of Pfizer's vaccine for children age 12 and older. It is the only vaccine to be approved for those younger than 18 in the U.S. Federal regulators declared the Pfizer vaccine is safe and offers strong protection for younger teens based on testing of more than 2,000 U.S. volunteers ages 12 to 15. CDC advisers endorsed the use of the Pfizer vaccine in kids as young as 12 on May 12 and the CDC quickly accepted their recommendations. Some vaccine clinics across the Twin Cities have started offering appointments — and in some cases actual doses of COVID-19 vaccine — to younger teens.
Are the vaccines safe for pregnant or nursing women?
The CDC now recommends that pregnant people receive COVID-19 vaccinations after a study published in April found no safety concerns with Moderna and Pfizer vaccinations given during the third trimester of pregnancy. Pregnancy is listed as a high-risk condition that could make a woman eligible for vaccination, depending on her age and other potential high-risk conditions, according to Minnesota Department of Health.
How much does it cost to get the vaccine?
Under Operation Warp Speed, the U.S. government contributed billions of dollars for the development of vaccines, and ordered hundreds of millions of doses. Federal health officials have pledged that vaccinations will be made available free of charge to all Americans. The Minnesota Department of Health has confirmed that vaccines will be provided to people at no cost, though health insurers may be billed.
Will vaccinations be mandatory?
Health officials encourage people to get vaccinated and expect vaccinations to be available to everyone who wants one, but it will not be required by law. However, some employers may require their employees to be vaccinated.
The Associated Press contributed to this report. This post has been updated.

Want to share info with the Star Tribune? How to do it securely

'Safe recovery sites' would offer syringes, naloxone and more to people using drugs. The plan could be in peril.
New Minnesota GOP leaders seek peace with party's anti-establishment wing

Who is Republican Lisa Demuth, Minnesota's first House speaker of color?